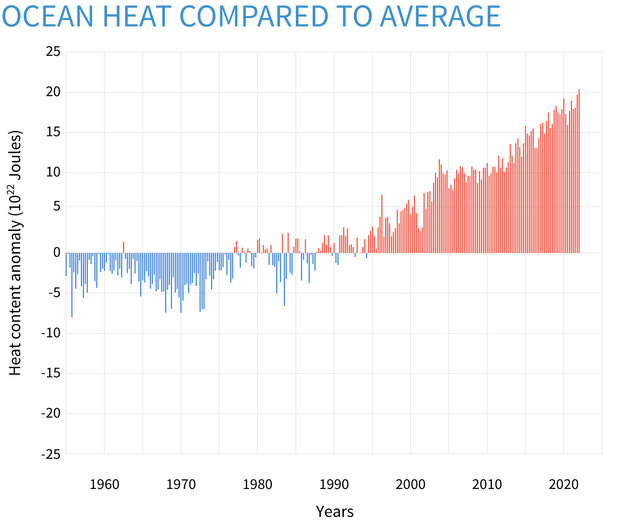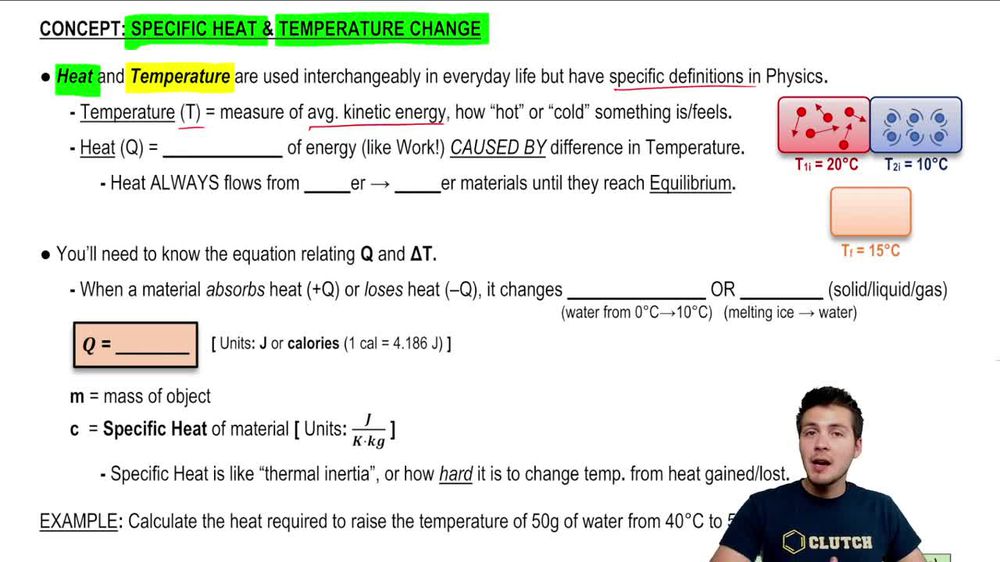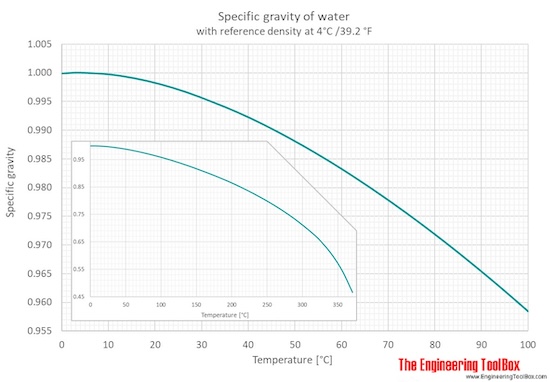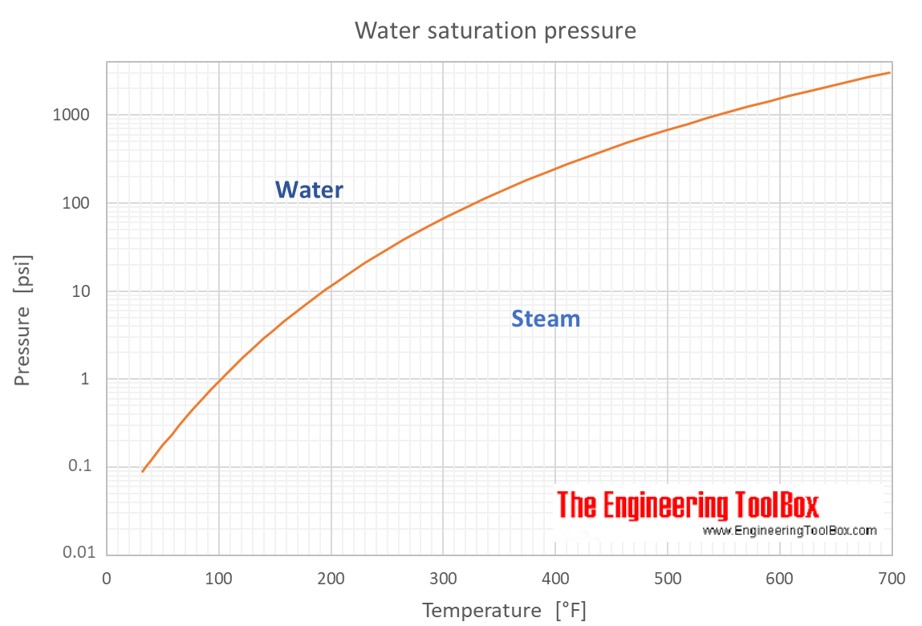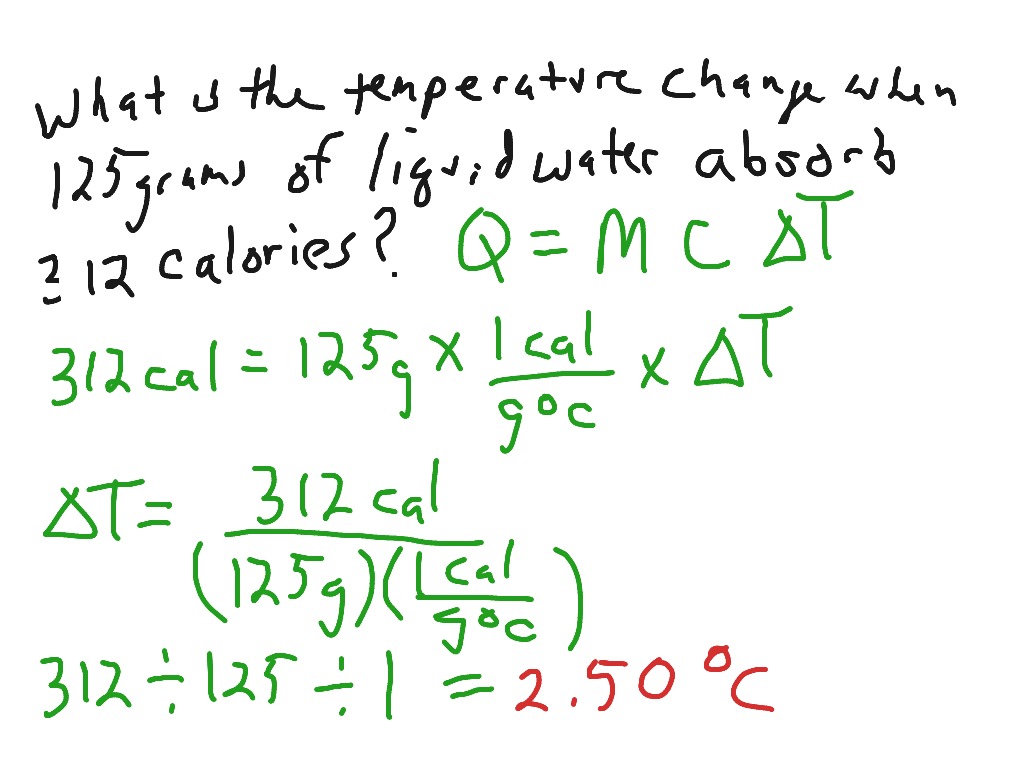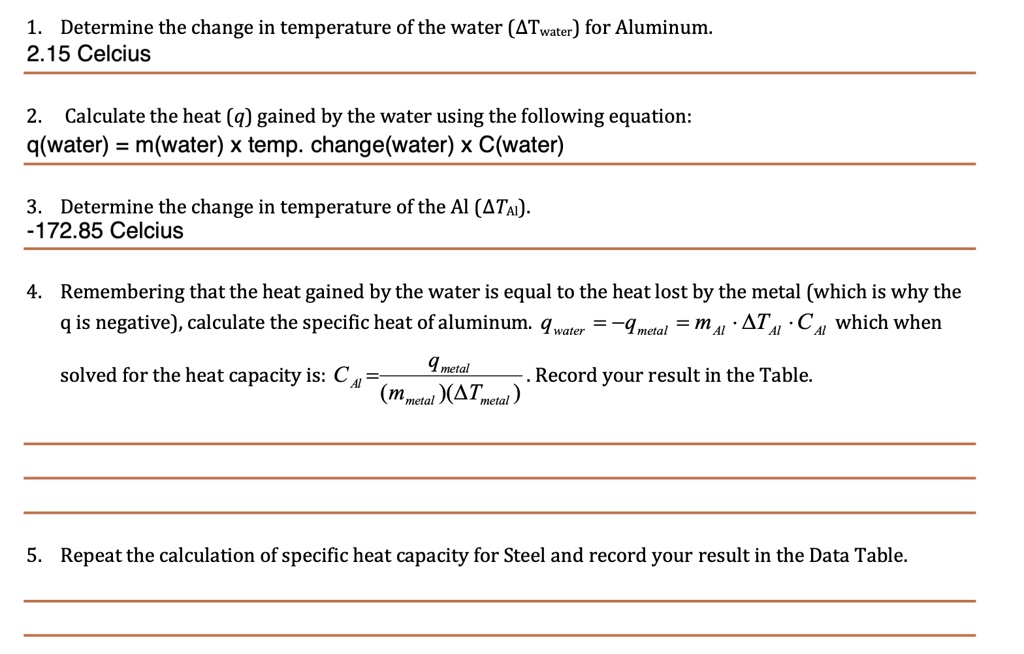
SOLVED: Determine the change in temperature of the water (ATwater) for Aluminum 2.15 Celcius Calculate the heat (q) gained by the water using the following equation: q(water) m(water) x temp. change(water) x
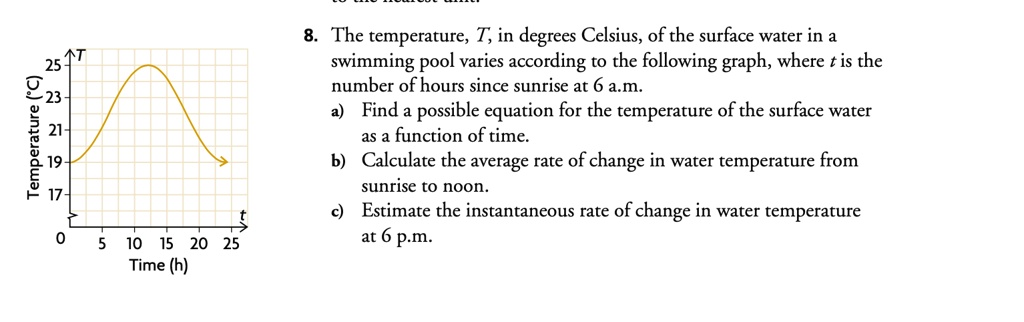
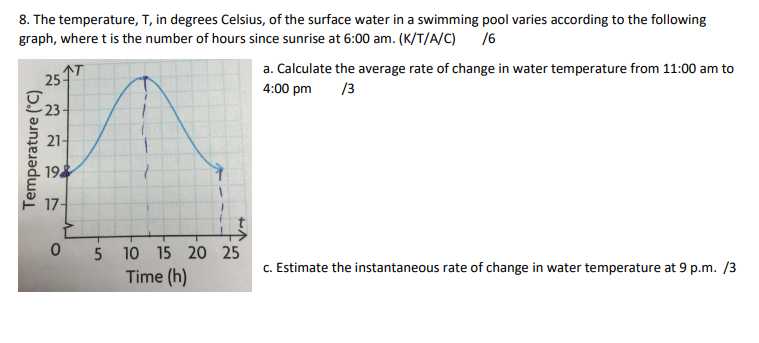
SOLVED: The temperature, T, in degrees Celsius, of the surface water in a swimming pool varies according to the following graph, where is the number of hours since sunrise at 6 a.m.

General Chemistry II - Transfer of Energy from Hot Metal to Water - Calculating Final Temperature - YouTube
How to calculate the rise in temperature when water falls from a height H, assuming that the whole energy due to fall is converted into heat energy - Quora
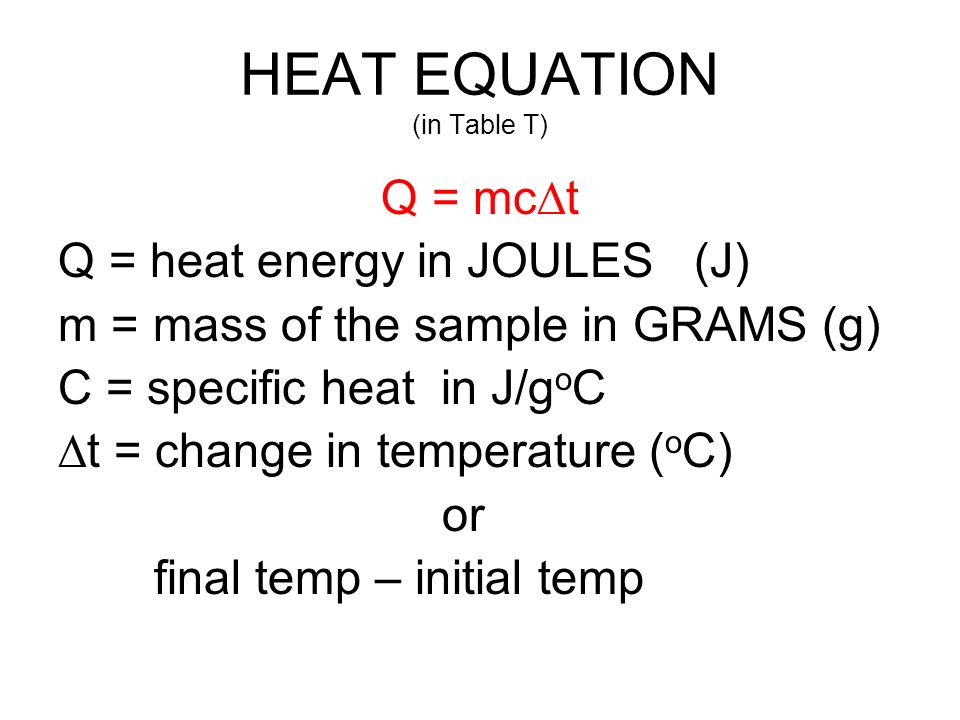
What mass of water will change its temperature by 3 C when 525 J of heat is added to it? The specific heat of water is 4-.8 J/gC | Socratic

Question Video: Calculating the Heat Energy Transferred to Water Using Its Specific Heat Capacity | Nagwa
![Calculate change in concentration of H^+ ion in one litre of water,when temperature changes from 298K to 310K . [Given Kw(298) = 10^-14, Kw(310) = 2.56 × 10^-14] . Calculate change in concentration of H^+ ion in one litre of water,when temperature changes from 298K to 310K . [Given Kw(298) = 10^-14, Kw(310) = 2.56 × 10^-14] .](https://dwes9vv9u0550.cloudfront.net/images/6961597/9d7c63c4-74c1-4469-8511-8aeadbc73da3.jpg)